Subsidy for Influenza Vaccination
Implementation overview
We provide full coverage for influenza vaccination costs.
* Vaccines not approved by the Ministry of Health, Labour and Welfare are not eligible for subsidy. This is because, in the event of adverse reactions, they would not be covered under the relief system based on the Immunization Act.
* Regarding the nasal spray vaccine “FluMist,” only products approved by the Ministry of Health, Labour and Welfare are eligible for subsidy. Product approved as of September 2025: FluMist Intranasal Spray (Daiichi Sankyo Co., Ltd.; Eligible age: Age 2 and above and under age 19). Please note that unapproved FluMist products are not eligible for subsidy.
* Depending on underlying medical conditions or health status, caution may be required for vaccination. Please be sure to consult with a physician before receiving the vaccination.
| Eligible Recipients (Vaccine Recipients) |
Insured persons and their dependents under the Society | |
|---|---|---|
| Subsidy Coverage Period (Vaccination Date) |
October 1, 2025 - February 28, 2026 | |
| Number of Subsidized Doses During Coverage Period | 1st | Age 13 and above (as of vaccination date) |
| 2nd | Age 13 and above (as of vaccination date) with underlying medical conditions (chronic diseases) where a physician has determined that two doses are necessary | |
| Under age 13 (as of the date of first dose) | ||
| Subsidy Amount | Full coverage | |
| Notes |
Vaccinations administered at medical institutions, local governments, and workplaces within Japan are eligible. Applications require a receipt containing the following information.
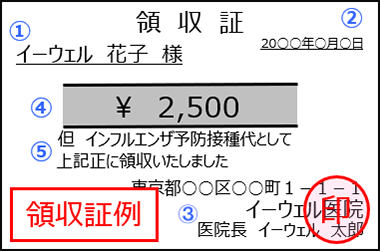 |
|